Peering into the brain with ultrasound
The Urban lab at NERF is transforming brain imaging through advanced functional ultrasound (fUS). Their latest series of studies reveals powerful new techniques to precisely map, visualize, and decode neural activity. Could these advances usher fUS into everyday neuroscience and clinical practice?
For decades, brain imaging has been defined by trade-offs. High-resolution optical imaging techniques like two-photon microscopy offer unparalleled detail but are constrained to small shallow regions of the brain and require invasive procedures. Functional MRI (fMRI) can map whole-brain activity, but at the cost of sluggish temporal resolution and reliance on bulky, expensive machinery. The deeper scientists probe into the brain, the more they must choose between scale, speed, and precision. These are compromises that continuously limit how we study neural dynamics, cognition, and disease.
Functional ultrasound (fUS) imaging is a technology that aims to change the equation. It is an emerging brain imaging technique utilizing Doppler ultrasound to measure tiny changes in cerebral blood flow that are correlated with neural activity. While measuring blood flow isn't new (fMRI is based on similar principles), what sets fUS apart is the extraordinary combination of speed, resolution, depth, and accessibility it can achieve. fUS allows scientists to capture high-resolution, real-time activity across the brain, even in active animals.
This technique offers remarkable advantages: high spatial resolution down to 100 micrometers, temporal precision of less than a second, and the ability to image deep brain structures - all achieved without requiring radiation or invasive procedures. In experimental settings, fUS has successfully visualized brain activity in various species from mice to humans, revealing intricate patterns of neural activation during tasks ranging from sensory processing to complex behaviors. Yet despite its potential, the technology has faced limitations in accessibility, detail, and reliability, which may hinder its potential to become a mainstream tool for neuroscience and medical applications.
At NeuroElectronics Research Flanders (NERF), a research institute empowered by imec, KU Leuven, and VIB, the Urban Lab is working to bridge these gaps. Their mission is to develop faster, smaller, and more powerful fUS technology, making it a mainstream tool for neuroscience and clinical applications. Now, in a remarkable series of three consecutive publications, researchers from the Urban Lab have unveiled a comprehensive suite of AI-powered tools that promise to democratize this technology. These innovations address the entire fUS workflow—from precisely navigating within the brain, to reconstructing intricate vascular networks, to extracting subtle activity patterns that would otherwise remain hidden. Together, they represent not just incremental improvements, but a remarkable step forward in how we can study the living, active brain.
By making the complex simple and the inaccessible available, these tools may soon allow researchers and clinicians to peer into the brain's innermost workings with unprecedented clarity, potentially transforming our understanding of everything from basic neural circuits to complex neurological disorders.

AI-powered brain navigation
The first breakthrough, published in Heliyon, tackles a fundamental challenge in brain imaging: knowing precisely where you are. The researchers developed a deep learning classification framework that can determine the exact anatomical position from a single micro-Doppler ultrasound image with remarkable precision: about 176 micrometers, roughly the width of two human hairs.
What this research establishes is essentially a GPS for the brain. Till now, traditional methods have required extensive expertise in neuroanatomy and time-consuming manual registration to reference atlases. The Urban lab’s AI approach automates this process, making it accessible to researchers without specialized training.
What makes this system particularly valuable is its reliability even in challenging conditions. When tested on brains affected by stroke, a condition where normal anatomy might be significantly altered, the AI maintained its accuracy, demonstrating robust performance even with brain vasculature severely impaired.
This navigation tool fundamentally changes who can effectively use fUS technology, opening the door for broader adoption across neuroscience labs and potentially clinical settings where precise anatomical localization is critical. As the neuroscience community increasingly adopts fUS, larger and more diverse datasets will become available, allowing these systems to become ever more widely applicable. Clinical translation, particularly in neurosurgery and neonatal imaging, represents a logical next step, where the ability to precisely navigate within the brain could enhance patient care.
Peering into the brain’s vascular highways
Building on this navigational foundation, the second publication in Neuroinformatics introduces an innovative approach to extract, visualize, and analyze the brain's vascular architecture. The vascular system is made up of blood vessels that run through the brain delivering oxygen and nutrients, and carrying away waste in a system so intricate that a single cubic millimeter of brain tissue contains hundreds of tiny capillaries. When something goes wrong, from a clot blocking artery, to a tiny vessel degrading with age to a tumor hijacking blood flow, the consequences can be profound.
Despite its importance, mapping this vascular network has remained a challenge. MRI and CT scans can capture broad structures, but they often necessitate the use of contrast agents, lack adequate resolution, and don't have the real-time capabilities needed to study minute changes in blood flow.
To break past these limitations, the Urban Lab refined µDoppler ultrasound imaging, adapting it to reconstruct the brain’s vascular architecture in three dimensions with sub-millimeter precision. The approach automatically segments blood vessels from ultrasound data, reconstructs them in three dimensions, and even classifies them as arteries or veins based on blood flow direction. Their method enables the visualization of over 1500 individual blood vessels per animal, resolving structures as small as 100 microns.
3D volume-rendered skeleton of flow-discriminated cortical arteries (red) and veins (blue):
This ‘digital twin’ of the brain's vascular network allows for easier quantification of measurements critical to understand cerebrovascular health including vessel density, length, and distribution. Unlike most available strategies that require contrast agents or invasive methods, this method works in vivo and can be completed within minutes.
The significance became clear when applied to a stroke model, where the technique precisely visualized the ischemic territory and quantified vessel loss. This advancement extends functional ultrasound beyond measuring brain activity to comprehensive vascular assessment, opening new possibilities for studying conditions like stroke, vascular cognitive impairment, and brain tumors.
3D volume-rendered skeleton before and after cortical stroke:
Advanced signal analysis to crack the neural activity code
The intricate brain vasculature is in place to support the neural activity of the brain: the electrical signals that race across circuits, surging in response to stimuli and quieting down in moments of rest. The key challenge in neuroscience is decoding the patterns of neural activity and making sense of how they correlate with perception, processing, and behavior. Functional ultrasound can offer a powerful window into this process, by measuring subtle blood flow changes that accompany neuronal firing.
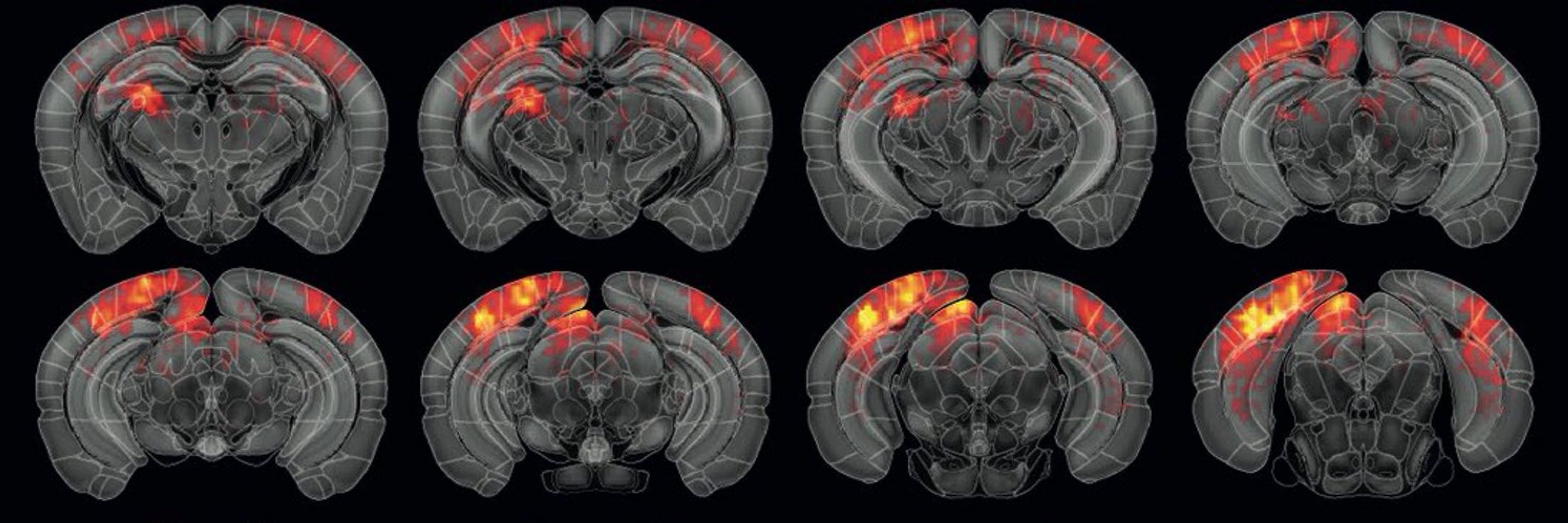
But current methods of fUS signal analysis make extracting meaningful insights difficult. Traditional analysis methods either average signals across entire brain regions (losing spatial detail) or correlate them with experimental events (missing unexpected activity patterns). To break free from these constraints, the Urban Lab introduced single-voxel clustering, a sophisticated method that extracts maximum information from fUS signals at the highest possible resolution, voxel by voxel, without assumptions.
When tested on visual responses in mice, this approach revealed subtle brain activity patterns that conventional methods missed entirely. At very low contrast levels where traditional analysis showed minimal response, single-voxel clustering identified clear patterns of neural activity with distinct spatial organization.

Altogether now
As functional ultrasound continues to gain traction as a neuroimaging modality, the Urban Lab's trio of publications represents a comprehensive approach to overcoming its technical challenges. By combining deep learning, advanced image processing, and sophisticated signal analysis, they've created a toolkit that makes this powerful technology more accessible, informative, and versatile. The navigation tool ensures precise positioning, the vascular analysis provides structural context, and the signal analysis extracts maximum functional information—each addressing a different barrier to the technology's broader adoption and application.
Importantly, the Urban Lab has made these tools available through open-source software, democratizing access to advanced neuroimaging methods. This approach reflects a commitment to accelerating scientific progress through collaborative innovation rather than proprietary technologies.
The potential applications extend far beyond basic neuroscience. These tools could enhance stroke diagnosis and monitoring, improve understanding of neurodegenerative diseases, and eventually support surgical planning where precise mapping of brain function and vasculature is essential.
What we're seeing is the transformation of functional ultrasound from a specialized research tool to a broadly applicable platform for understanding the brain. For researchers and clinicians interested in peering into the brain's complex operations, that transformation couldn't come at a better time. As neuroscience tackles increasingly complex questions about cognition, emotion, and disease, these new tools may provide exactly the window we need to see the answers.
OpenfUS: a nexus for global users of fUS technologyWant to be kept up-to-date on our biotechnological news and stories? Join our community and subscribe to our bi-monthly newsletter.
Vinoy Vijayan