The remarkable ambition to map 37,200 billion cells in the human body
More than 3,000 scientists are busy building an atlas of every cell in our body. This should point the way to new medical breakthroughs. Like in a lab in Ghent, where they want to grow immune cells with an artificial organ to fight cancer.
This article was written by Stephanie De Smedt for De Tijd.
Before a heart surgeon at Ghent University Hospital begins his delicate task on a child, he must first remove a piece of tissue that lies like a soft cap over his young patient's heart. In the past, that piece would have been lost, but today it is given a second life in science. The surgeon places the tissue in a capsule and via an underground pneumatic tube system, it ends up in the laboratory of biotechnology professor Tom Taghon, a few buildings away, five minutes later. 'At least, if the parents give permission', says Taghon.
What happens to the tissue fits into one of the most ambitious scientific projects ever. More than 3,000 scientists from 86 countries around the world are working on mapping every cell in the human body. Never before have researchers attempted to create such a detailed blueprint of our body, cell by cell, tissue by tissue. The Human Cell Atlas (HCA) should not only drastically improve our understanding of the body, it should also trigger a revolution in medicine.
In Ghent, it starts with the pneumatic tube. Taghon shows a transparent dish on a lab table. In it lies a soft, pale lump of a few centimetres in size, interwoven with red blood vessels. It is a piece of the thymus, also known colloquially as the sweetbread. The organ is particularly large in the first years of life, which is why the surgeon has to remove a piece of it from young children to better reach the heart. As we get older, it loses its function and shrivels up.
Training centre
For centuries, the thymus was seen as a mistake of evolution, a piece of useless fat. But since the 1960s, we have known that the organ determines how our immune system works throughout our lives. It is the training centre for the T cells, where the T stands for thymus. The elite troops among the white blood cells protect us against viruses, bacteria and even cancer cells.
"It really works like a school," says Taghon. Blood stem cells from the bone marrow end up in the thymus. And just as students have to go through different school years, these immature immune cells are trained in stages. They learn to leave the body's own cells alone and attack intruders. Only the best ones succeed and are released into the bloodstream to protect the body.
The dream is a ready-to-use cell therapy that is available to everyone, without long waiting times and high costs.
We know that the thymus trains T cells in this way, but we still don't know how it does it. If scientists crack that code, it would open the door to a new era of immunotherapy, in which we can specifically reprogram and even rebuild our immune system. Autoimmune diseases, in which the immune system goes off the rails and attacks the body itself, would be easier to treat. Vaccines and infection control would become more precise. Taghon's ultimate goal is to build an artificial thymus, a biological factory that produces immune cells in the lab.
"T cells are already used in therapies such as CAR-T to fight cancer," he says. A little further on, in Zwijnaarde, Legend Biotech and Johnson & Johnson are building a high-tech site for such sophisticated treatments. For some cancers, such as blood cancer, this yields promising results. "But it is expensive and time-consuming. T cells are taken from the patient's blood, genetically modified and returned. Because the original cells come from a sick patient, the quality is not always optimal."
The dream is a ready-made cell therapy that is available to everyone, without long waiting times and high costs. "That would mean a huge step forward for immunotherapy. It would also help to give stem cell transplants in leukemia treatments a push in the right direction."
Money and know-how
To realize that dream, Taghon's lab must be able to map the entire thymus precisely. That led the immunologist to a bioinformatician: Yvan Saeys, professor of machine learning at VIB. "If you want to reconstruct an organ, you have to know exactly which cells are in it, what their function is and how they communicate with each other to perform that function. That's where we come in," says Saeys.

"You have to examine millions of cells and collect thousands of parameters per cell. You can no longer do that by hand or with a microscope. The amount of data is too large and too complex. We need computer models to analyze everything. And the computing power of a supercomputer." What Taghon and Saeys wanted to do, they could not do alone. They had neither the money nor the know-how to do everything themselves. That is how they ended up at the Human Cell Atlas, an international project that was started in 2016 by the German bioinformatician Sarah Teichmann, professor at the University of Cambridge, and the Israeli biologist Aviv Regev, today head of research at the American biotech giant Genentech.
Just like Google Maps, you could zoom in and out of the cell atlas. You could navigate based on the map: how do hormones travel through the body?
Teichmann and Regev thought it should be possible to build a kind of Google Maps of our body. "An atlas that not only shows which cells there are, but also where they are and how they work together," says Teichmann from Cambridge. "Just like with Google Maps, you could zoom in to street level, but also zoom out to see an entire city at once. You could navigate based on the map: how do hormones travel through the body? How do organs communicate?"
The emphasis of the cell atlas is understanding the body in a healthy state. Teichmann: "We often have to explain that well to our financial backers. A lot of funding goes to disease research, such as cancer studies. We want to map the healthy reference, because you first have to know how a healthy body works, before you can understand what changes in disease."
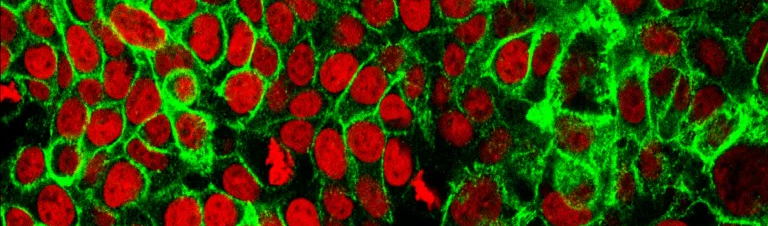
The idea for the atlas came after several technological breakthroughs. In science, people sometimes talk about the omics revolution, referring to the suffix of a number of disciplines for biological data analysis that have made spectacular progress since 2009. With so-called single cell genomics, scientists could study a single cell, instead of looking at large groups. Spatial genomics made it possible to know in detail where each cell is located in the body. With transcriptomics, they could see which genes are switched on or off in a cell.
"We realized that it could be something big," says Teichmann. "We were clearly entering a new era of cell biology. We could now take a piece of human tissue - from the skin, the heart, or the brain - and determine exactly which cells were in it. If you could do that for the entire body, that would provide completely new insights."
For more about spatial omics, check out our introductory blog!
More cells than stars
The idea sounded logical, but the task was enormous. Our bodies contain no fewer than 37,200 billion cells, far more than there are stars in our Milky Way galaxy. Mapping them all required biologists, doctors, computer scientists, mathematicians, and engineers. "We also absolutely wanted to work with tissue samples from all over the world, and not just samples from rich, Western countries," says Teichmann. "People everywhere have different susceptibilities to diseases. Race, gender, diet, and lifestyle can make a difference. We wanted to understand that."
It had to become an international collaboration. In October 2016, the two initiators organised a first meeting in London. There were 93 scientists. That number grew every year, until it became the largest scientific consortium ever. In 2019, Taghon and Saeys joined.
"The big advantage of the HCA was that we as researchers could move much faster and work on a much larger scale," says Saeys. "The project turbocharged the further development of all the technologies we needed. In the beginning, we could analyse 10,000 cells per sample, now that number is millions. We no longer have to chop the tissue to pulp to see which cell types are in it, we can work with tissue slices. Recently, we have also been able to spatially analyse where the cells are located."
The laboratory techniques have improved, the resolution of the imaging has been refined, robots help to process more samples at the same time, and powerful algorithms help with the analysis. "We can now collect a lot more information from the same piece of tissue. In the Google Maps analogy, it's like we can not only see the houses, but also look inside to see where the cabinets are," says Saeys.
37,200 billion cells
Our body contains no less than 37,200 billion cells, far more than there are stars in our Milky Way galaxy.
Ozempic
In 2020, Saeys, Taghon and Teichmann, together with a few other researchers, built a first atlas of the thymus. At least, a version that defined all types of cells. An update followed last year, in which a start was already made on spatial analysis: which cells are located where?
"The thymus was probably one of the first complete atlases of an entire organ," says Teichmann. "There are now 18, including those of the heart, lungs, kidneys, brain and skin. Next year we want to publish the first real version of the entire HCA. It will be largely complete as far as the individual cells in our body are concerned. But when it comes to spatial information, i.e. where exactly the cells are located and how they work together, we are only at the beginning. Also for the thymus. That will require at least another five years of work."
If we understand how each cell works, we will be much better able to predict how drugs will work and how to prevent side effects.
In the meantime, new insights are piling up. "In every new tissue that we investigate, we find new surprises," says Teichmann. One of the most exciting discoveries she made together with Taghon and Saeys: in the thymus of unborn babies, they found a completely new type of T cell. "These cells only seem to occur in the thymus during pregnancy. They work much faster and more generally than normal T cells. That can give us clues about how we can better control the immune system, for example to better fight infections or treat autoimmune diseases."
"Our understanding of our body was actually very limited. Wikipedia states that there are a few hundred cell types in the human body. The cell atlas shows that there are probably between 5,000 and 10,000. A huge difference," says Teichmann.
"Even of cells that we already knew, we only now understand how they work. Take the pacemaker cells, which regulate the heart rhythm. We knew that they make the heart beat faster or slower via chemical signals, such as adrenaline. But now we have discovered that there is another way of communicating. The cells also have a certain receptor, a switch that helps determine the rhythm."
"It's the same receptor that drugs for obesity and diabetes, like Wegovy and Ozempic, work on. That explains why people taking those drugs see their heart rate increase by six beats per minute. Until now, we had no idea why that was. If we understand how each cell works, we will be much better able to predict how drugs work and how we can prevent side effects."
Teichmann calls the plan to build the cell atlas 'more ambitious' than the prestigious Human Genome Project, the international initiative that first mapped our entire DNA in 2003. "The Human Genome Project gave us the book of life, our work should show how that book is read in the body."
It is not only bigger, but also more impactful, she believes. "With the Human Genome Project, it took years for the first insights to become really useful. The genetic data was published around 2000, but it took another decade before real connections between genes and diseases could be made. With the cell atlas, even the first rough work already has a big impact."
That became clear during the corona pandemic. Thanks to the gigantic dataset of the cell atlas, scientists quickly knew which cells had the entry gates for the virus. "They were in the eyes, nose, mouth and salivary glands," says Teichmann. "That helped to understand how the virus spread through the respiratory tract and saliva, and why face masks were necessary."
Organoid
In the lab in Ghent, we see on the screen of a microscope an image of something between an egg yolk and a brown bean. It is an enlargement of an organoid that in reality is not even the size of a pinhead, barely visible to the naked eye. It is a highly simplified miniature version of the thymus that Taghon's team has grown based on the insights from the cell atlas.
"To make an organoid like this, we bring together stem cells with a few supporting cells," a staff member explains. She points to a dot outside the orange-brown core. "The supporting cells give the stem cells signals to develop into T cells. That takes a month or two. Here you see one coming out. You start with a small number of cells, but tens of thousands of descendants come from one cell."
"So the system works," says Taghon. "But the cells we produce are not at all as efficient as normal T cells. In a real thymus, dozens of cell types play a role, we currently only use one. We do not yet know enough to perfectly mimic the process."
Understanding how all the cells in the thymus work together is also just one step. The next step is to map out how the T cells sent out by the thymus adapt to the place where they end up. Taghon is one of the coordinators of an international project that is trying to visualize the entire immune cell network. "But that requires an enormous amount of data," he says. "An entire career's work."
"It is an incredibly exciting time," says Teichmann. "In the past, we had to use fruit flies, worms and mice as model systems. This is the era of human biology. Finally, we can now study humans in depth. That is absolutely mind-blowing. It's incredible how ingenious our bodies are."
Millions for Belgian pioneering work
Because the Human Cell Atlas (HCA) focuses so heavily on international collaboration, the project is particularly cost-efficient. "The Human Genome Project has invested 3 billion dollars. In our case, it is more like a few hundred million," says co-founder Sarah Teichmann.
The consortium of now more than 3,000 members from over 1,000 institutes worldwide shares resources, expertise and infrastructure. The grants that one lab receives help the work of other researchers advance. According to Ghent professor of machine learning Yvan Saeys, a member of the HCA, this has in turn set a catalyst for the development of better and therefore more cost-efficient techniques.
The HCA receives support from philanthropic institutions such as the Chan Zuckerberg Initiative (from Facebook founder Mark Zuckerberg and his wife) and the Wellcome Trust (from the estate of the British founder of the predecessor of GlaxoSmithKline) and from research programs in Europe and the United States, among others. Money also comes in through donations from individuals. And recently there was also a pharmaceutical forum, where (now three) large pharmaceutical companies pay an annual contribution of 100,000 dollars to gain access to meetings, scientists and technology. The data are in principle freely available to everyone.
The Ghent research has now received more than 1 million dollars from the Chan Zuckerberg Initiative. "That opens doors," says Saeys. "Last year we secured a 2 million euro project to develop new single-cell technologies. We are now pioneering that field in Belgium."
.jpg.webp)


