How sick ‘nurses’ in our central nervous system play a role in ALS pathology
This blog originally appeared in Dutch in EOS Wetenschap.
Imagine that in a hospital, not only the patients but also the nurses become seriously ill. That's what happens in the bodies of amyotrophic lateral sclerosis (ALS) patients. In my PhD in the Laboratory of Neurobiology at the VIB-KU Leuven Center for Brain & Disease Research, I investigate what goes wrong with astrocytes, the 'nurses' of our nervous system.
21 June, World ALS Day, our lab is full of ALS patients and their loved ones, who come to visit our research group. ALS Liga, the Belgium ALS patient association, organizes this great initiative where we try to provide insight into our research on the disease that affects them. However, it can also feel a bit challenging, especially when a man whose ability to speak has been taken away by ALS shows me a question on his mobile phone asking whether we are conducting research on advanced ALS in our lab. How do I tell them how complicated this terrible, fatal disease - with which they or their loved one has been diagnosed – really is? There are currently 1,000 ALS patients in Belgium and more than 400,000 worldwide. The lack of a drug for these patients is most likely due to the complexity of the disease. Why is this disease so complicated?
The complexity of ALS
Let me start with what we know. We know that ALS attacks the motor neurons in our nervous system. Motor neurons are the nerve cells that connect with and control our muscles to ensure we can walk, eat, talk, and so on. When the motor neurons die, the connection between these nerve cells and the muscles is lost, which results in paralysis, causing ALS patients to end up in a wheelchair and having increasing difficulties with eating and speaking. Finally, the disease also attacks the motor neurons that connect to the respiratory muscle, causing patients to die due to respiratory failure. The average lifespan after diagnosis is three to five years.
However, if we look at one of the most famous ALS patients - British physicist Stephen Hawking - we can see that this is not always the case. Living an additional 55 years after his diagnosis, Stephen was one of the longest-living ALS patients and is a perfect example of the unpredictability of the disease. Not only can the duration of the disease vary, but the age at which the disease develops, the clinical picture, and the cause (genetic or not) differ between patients. Are you starting to see just how complex it is? Well, it becomes even more complicated when I tell you that it's not just the motor neurons that are failing…

Sick nurses and wrong medication
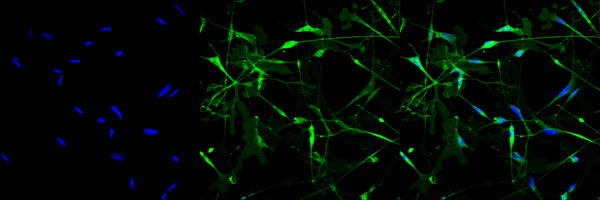
Imagine that in a hospital, not only the patients are sick, but the nurses too. This would certainly not benefit the health of these patients! But that's pretty much what happens in ALS. Imagine motor neurons as patients who get sick and, unfortunately, die. Then, imagine astrocytes - star-shaped cells that support neurons - as the nurses of our nervous system. Research has shown that these “nurses”, besides the “patients”, also become ill in people with ALS. In fact, not only do these nurses fail to take care of the patients but, to make matters even worse, also administer the wrong medications!
Astrocytes have various crucial functions in our nervous system. For example, they support the formation of connections between nerve cells, aid signal transmission, provide nutrients, help with cleaning up, and support blood vessels in protecting against harmful substances. It’s clear that the role of astrocytes in a healthy brain should not be taken for granted! You can imagine how a disturbance in the normal functioning of astrocytes might affect the health of nerve cells. This appears to be the case in ALS.
In the Laboratory of Neurobiology at the VIB-KU Leuven Center for Brain & Disease Research, we discovered that ALS-linked genetic defects disrupt the normal homeostasis of astrocytes and that these cells even secrete substances that can be toxic to motor neurons (1). Furthermore, if we add these 'sick' astrocytes to motor neurons, we’ve found that this disrupts the outgrowth of motor neurons and the formation of their connection with muscle cells. These processes are both hallmarks of ALS, so we can conclude that ALS-affected astrocytes influence the health of motor neurons through the loss of their supportive function and the gain of neurotoxic effects.
Does this mean that we were completely wrong by focusing our research on the motor neurons and that astrocytes are actually the culprits? No, it's not that black-and-white. We still see that things also go wrong in the motor neurons in the absence of astrocyte interference. After all, motor neurons are still the “patients” of our hospital, but the sick “nurses” certainly also play a crucial role in helping or hindering the health of these patients.

(1) FUS-ALS hiPSC-derived astrocytes impair human motor units through both gain-of-toxicity and loss-of-support mechanisms. Stoklund Dittlau, et al. Mol Neurodegener, 2023. DOI: 10.1186/s13024-022-00591-3.
There is hope!
Based on the findings of our research and the research from other labs, it’s becoming evident that it is not enough to only focus on motor neurons if we want to find a cure for ALS. In a hospital with sick patients and nurses, doctors would also prescribe medication for both of them if they want everyone to be healthy again.
Fortunately, there are now new scientific tools available to investigate the effect of possible new treatments on different cell types. For example, in our lab, we use induced pluripotent stem cells (iPSCs). iPSCs are stem cells created from patient skin cell samples that can be used to make any other cell type in the body, including motor neurons and astrocytes. As demonstrated in the study described above, these enable us to study the effect of ALS on motor neurons and astrocytes from ALS patients, which is something that was not possible before. Another technique we are now using is single-cell RNA sequencing, a recent technological advancement that allows us to read the RNA of each cell, derived from tissue from patients or mouse models, individually. This allows us to study ALS in more depth than scientists were able to before.
Both methods provide the opportunity to study what goes wrong in different cell types in ALS and, on top of that, what the effects of possible treatments are on these different cell types. These new insights are promising and can hopefully contribute to the discovery of a treatment for ALS. I’m hopeful that there will be a day when we can invite ALS patients and their loved ones to our lab again and show them how our efforts - and those of ALS scientists around the world - finally led to the cure for ALS.
Christine Germeys
Want to be kept up-to-date on our biotechnological news and stories? Join our community and subscribe to our bi-monthly newsletter.
Related stories